
Finding the right ELISA plate
reader manufacturer in India is a big decision for any lab. A good plate
reader gives reliable results. A bad one slows down your work and adds risk. If
your lab runs ELISA tests often, you can’t afford to guess.

Finding the right ELISA plate
reader manufacturer in India is a big decision for any lab. A good plate
reader gives reliable results. A bad one slows down your work and adds risk. If
your lab runs ELISA tests often, you can’t afford to guess.

Webinar about Immunomodulation by Food to Maintain Gastrointestinal Health on Thursday, February 13, 2025, at 8:30 PM (IST).

In this testimony, we intend to unlock the perceptivity and competence in Gel Electrophoresis.

Compared to existing options, it is ten times more effective.

The goal is to create an oral medication by identifying small molecule inhibitors of Sclerostin.

In this tale, we confer about the critical role of DNA ladders diverse applications in Molecular Biology.

The number of bacteria in the brain was decreased by almost 1,000 times when nano-aggregates were delivered via the nose compared to mice that were not treated.

Hydrogels have been described to be drug carriers owing to their swelling properties
Source: BioSpectrum
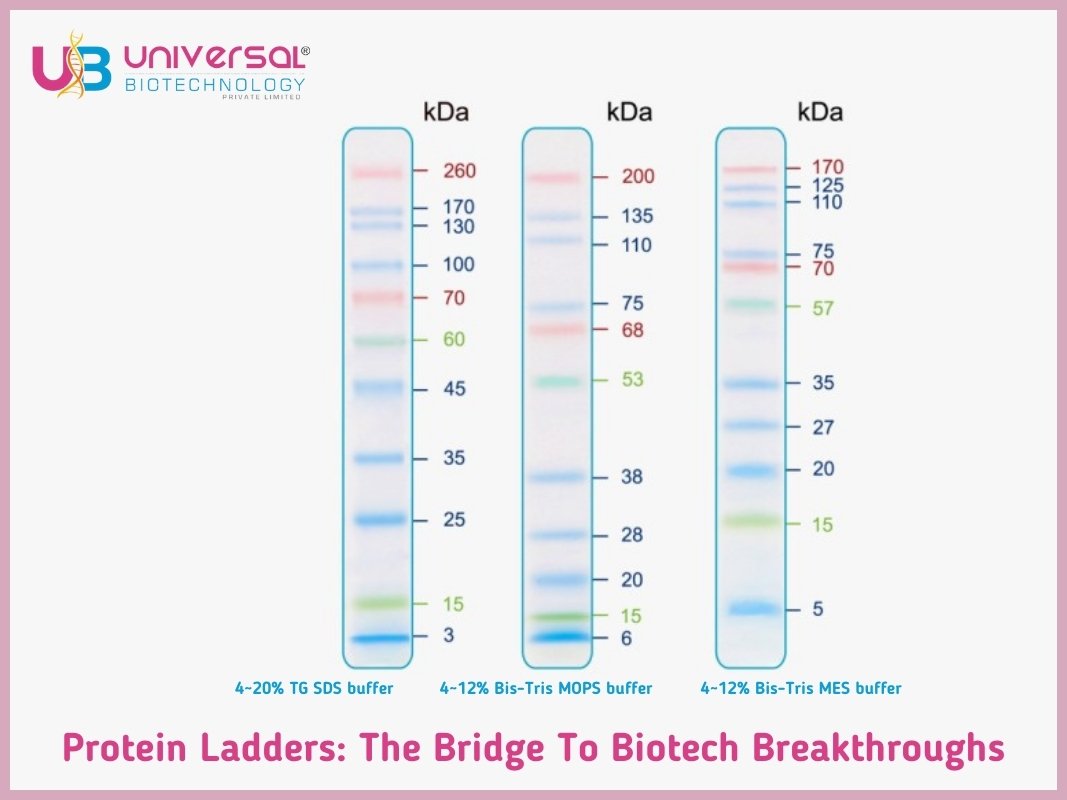
In this chronology, we confer about the significance, composition, application and other segments of protein ladder in Biotechnology advancement.
.jpg)
The projected budget for executing the integrated program 'Bio-RIDE' is Rs 9197 crore.
Source: BioSpectrum

In this narration, we will discuss about the principle behind transilluminators with a special focus on LED Transilluminators and their advantages. We will also look into their applications, target audience, and future prospects.

To ensure HILLCHOL is both accessible and affordable for nations facing the highest disease burden.
Source: BioSpectrum

Shri Priyank Kharge leads debate inviting tech leaders, industry associations, startup founders and media to Bengaluru Tech Summit as Asia’s largest tech show.
Source: BioSpectrum

In this journal, we glance upon Estradiol hormone production, regulation, function, disorder and Biotechnology research. Examine the food safety kits with us from renowned brand Elabscience to detect the Estradiol hormone residues samples.

This blog provides In-Depth Capsule on centrifuge, highlighting on their precept of operation, procedure and applications in several realms with Universal Biotechnology Private Limited. Explore the centrifuge from DLAB Scientific.

A professor of plant Molecular Biology needs individuals to know that plants have stem cells as well. Fair like in the therapeutic world, plant stem cells might bolster human development and improvement when utilized to make strides the nourishment supply. The researcher's lab found a transcription factor gene called HVA that controls cell division in vascular stem cells.

Analysts genetically modified poplar trees to produce high-performance, structural wood without the use of chemicals or energy intensive processing.
Source: ScienceDaily
.jpeg)
This blog provides a comprehensive overview of enzymes, touching on their structure, function, and significance in various domains with Universal Biotechnology Private Limited. Explore the Research Use Only (RUO) enzymes tools from Abbkine and other overseas brands.

ELISA kit plays a crucial role in biomedical research, clinical diagnostics, and various industries. Their ease of use, sensitivity, and specificity make them indispensable tools for detecting and quantifying molecules of interest.
In this blog, let's dig about ELISA kit and
unveil the prospective with Universal Biotechnology Private Limited.
Explore the Research Use Only (RUO) ELISA kits from LabReCon
(Make in India brand) and other overseas brands.

In this chronicle, let's delve into the revelation of Biotechnology power from the production of pharmaceuticals, biofuels to the manipulation of genetic material, shedding light on its applications, advancements, ethical considerations and its continuous expanding scopes.

Uncover the Genomic Research on Cardiovascular Health and its Genetic Predispositions, Risk Prediction, Prevention, Drug Development and other considerations.
In this blog, we embark on a journey through the transformative impact of genomic research on understanding, managing, and potentially eradicating cardiovascular diseases.
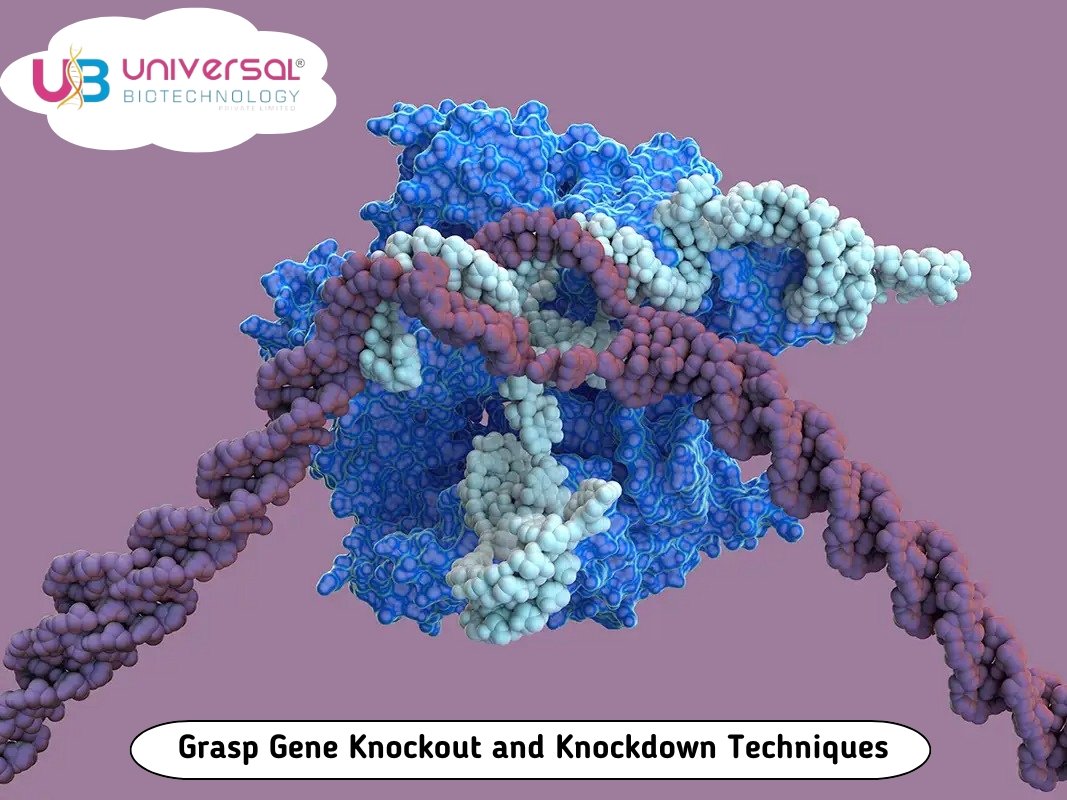
Gene knockout and knockdown are essential techniques in molecular biology used to study the function of genes.
In this article, we'll delve into the principles, methods, and applications of gene knockout and knockdown.
.jpg)
Date: 18th May, 2024
Venues: UBPL Corporate Office, Rajouri Garden, and Duty Free-Flaunt at
Vegas Dwarka, New Delhi.
Key Insights: Understanding Thromboxane B2 (TXB2) and Its Applications in Research and Diagnostics....
Thromboxane B2 (TXB2) stands as a pivotal metabolite in the realm of eicosanoids, derived from arachidonic acid and intimately linked with platelet function and wound healing. Its inert nature makes it a crucial marker in understanding platelet activation, vascular health, and the efficacy of anti-coagulant therapies. In this comprehensive exploration, we shed light on the role of TXB2, its detection methods, and the revolutionary Arbor Assays-Thromboxane B2 (TXB2) ELISA Kit, offered by Universal Biotechnology Private Ltd.
Universal Biotechnology Private Limited, your trusted partner in Research and Clinical Diagnostics. Our aim to provide solution to our customer with "One Stop Infinity Choices" motto, we understand the significance of these versatile tools in propelling scientific discovery and improving healthcare outcomes.

Expert Strategies: Troubleshoot Your Elisa Assay with Confidence and Ease....
Universal Biotechnology Private Limited, your trusted partner in the world of biotechnology and research. ELISA (Enzyme-Linked Immunosorbent Assay) kits where we embark on a journey to master the art of troubleshooting Elisa assays for unparalleled success in your research endeavors. At Universal Biotechnology, we understand the challenges researchers face in optimizing their experiments, and we're here to guide you through the process of troubleshooting your Elisa assay effectively. As your "One Stop Infinity Choices" supplier for ELISA kits in India, we understand the value of your each sample and accurate result.
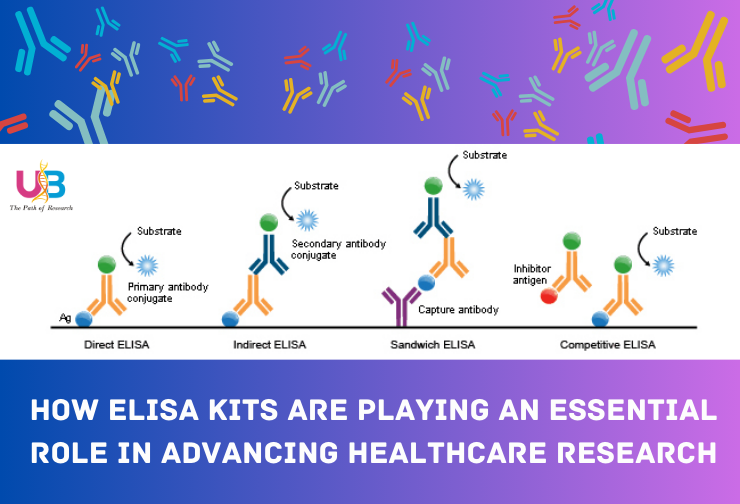
ELISA Kits: The Catalyst for Healthcare Research Advancements....
Universal Biotechnology Private Limited, your trusted partner in the world of biotechnology and research. ELISA (Enzyme-Linked Immunosorbent Assay) kits play in advancing healthcare research. As your "One Stop Infinity Choices" supplier for ELISA kits, we understand the significance of these versatile tools in propelling scientific discovery and improving healthcare outcomes.

Empowering Indian Research with Exceptional Antibodies....
Universal Biotechnology: Your Gateway to a World of Antibodies, Unmatched Variety, Unbeatable Solutions "One Stop, Infinity Choices"!!!!
.png)
Looking for High Quality & Sensitivity an ELISA kit in India?
Now became so easy with Universal Biotechnology Private Limited, The only company with “One Stop Infinity Choices” for all your ELISA Kit requirements.
.png)
SARS-CoV-2 Omicron therapeutics: Getting to know the unknown and the road ahead on Wednesday, Feb 22, 2023, at 10:00 p.m. (IST)

Blood plasma-derived extracellular vesicles: A minimally invasive tool for predicting response to cancer treatment on Tuesday, Nov 29, 2022, at 08:30 p.m. (IST)

Restoring stem cell fate and behavior outside their native
niche on Thu, June 28, 2022 7:30 PM (IST)

The SARS-CoV-2 RNA-protein interactome at subgenome resolution on Thu, Apr 21, 2022 7:30 PM - 9:00 PM IST

Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes.